If Lucid ever reaches space, this is some brainstorming of the potential logo
Latest News and Social Media Posts

Case Study: Cell Cultured Seafood Remote Visualization and Historian
1 | Executive Summary Accelerating Lights Out Cultivated Seafood Production with Mobile Data Infrastructure Wildtype Foods, a leader in cultivated seafood, engaged

Life is Too Short for Bad S88 Batch
Life is too short for poor batch implementations. Many organizations are purchasing equipment—especially bioreactors—with an inappropriate batch philosophy, resulting in

Custom Chromatography Cooling Fan Brackets
Fixing $1M OEM crappy design with a little flair… Who mounts a fanless industrial PC upside down in a pendant

FactoryTalk View SE, Historian SE Development Sprint
Not a flex, more of a Hail Mary in response to delayed shipping… I had engineers sitting around waiting for

VMWare ESXi vs Hyper-V
GMP Automation’s Creed: “This is my hypervisor. There are many like it, but this one is documented. My infrastructure is my life. Without

Allen Bradley 6300 Thin Client Mounting Bracket
Needed a vertical, 90‑degree DIN‑rail mount to retrofit a machine from a crappy PV+1000 to FT View SE thin client. While I
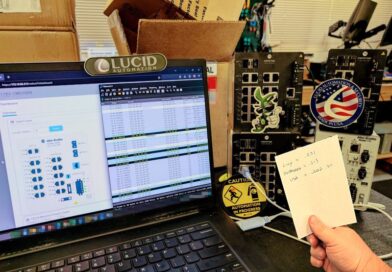
ITOT Switch Characterization – Lucid Lab
Doing some switch characterization tests in the lab. Glad we starting making magnets over stickers. Helps with naming devices and

Rack Mounted Industrial Switches
Putting together an order for two 19″ rack mounted Cisco IE-4000’s. This client is running out of wall space on

FactoryTalk Upgrade – Best of 2014 to Best of 2025
Finally got the go-ahead to upgrade a system I installed back in 2014, and the results are sweet. Zero downtime, more

Stay Compliant. Automate Smarter.
GMP compliance is time-consuming, don’t make it harder with a bolt-on solution. A turnkey system designed for easy documentation, validation,

Stratix 5700 vs 5200 – Lucid Thoughts
Is the Stratix 5200 an improvement over the old 5700? I’ve seen the early reviews from the folks preferring alternate
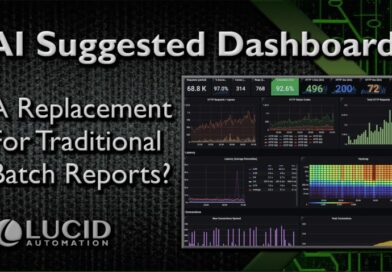
AI Suggested Dashboards
In GMP life sciences, upgrades are tricky with all the legacy validation to consider. Swapping out a reporting solution, the

GMP Process Data Lacking?
Nobody reads them, but our case studies show one thing: when Process and QA engineers start with a blank slate,

GMP Equipment FAT’s – Let’s Talk About It
GMP Equipment FATs, big issue… let’s talk about it. OEM’s have great sales teams, but we’ve been seeing the operations teams

AI in my PID Loops? – Automation World
https://www.automationworld.com/control/article/55279765/control-system-integrators-association-csia-ai-in-industrial-process-control-enhancing-the-pid-loop Wrote an article for Automation World about replacing PID loops with AI/ML outputs… Read link in comment before flaming

UL 508A HMI Retrofit – Parts are In!
Surprised with this stack in the shop. We’re working on a large UL 508A HMI retrofit, and the new Thin

Bioville 2025 – Solano Collage and Vacaville, CA
Great time yesterday at Solano Community College for their hashtag#Bioville event. The Lucid team was impressed by the facility tour.

Single-Use Chromatography and TFF is Our Specialty
Single-use systems get a bit of a bad rap. Not so much for the equipment itself, but because of the
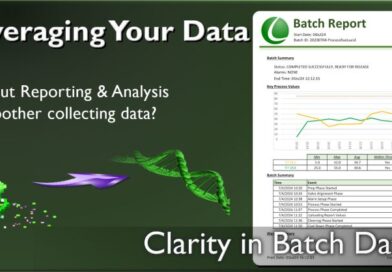
Leveraging Your Batch Data
It seems everyone has drunk the “Collect all the Data” kool-aid, but forgot the “Analyze and Report” chaser. One more raw,

ISPE Facility of the Future Finalist
Dive into the heart of innovation with our latest video, “4 Strategies for Automation-Driven ISPE Facility of the Future.” Discover

UC Davis AIChE Career Day
The Lucid Automation team had a fantastic time meeting with the UC Davis Chemical Engineering AIChE chapter and talking career
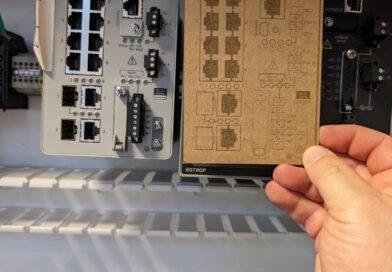
Second Laser Added to the Shop
Added second laser to the shop. So far, best application has been making dimensional-accurate cardboard cutouts for panel retrofit job

Allen Bradley PLC and DeltaV PK Controllers – Friends??
Friends? Probably not, but we’re pretty good making them play nice on a GMP factory floor. We just wrapped up

Lucidbrau Octoberfest
Oktoberfest was a great opportunity to introduce our new interns— Andrew, Kimberly, and Jordan—to the industry. The event was a

ISPE Women in Pharma Tennis Tournament
Proud sponsor of the first annual ISPE Women in Pharma Tennis Tournament! It was a fantastic event, well-executed at the

Official Logo Trademark
The Lucid Automation logo is now officially trademarked by the USPTO! For those considering the journey of securing a registered

How to Factory Test With Your Site Infrastructure
No equipment exists on an island. Sooner or later, process data and audit logs must connect to your facility’s infrastructure.

Front-Facing Ethernet Ports
Not sure if I dislike front-facing ethernet ports or those 6’ armored strain boots on some industrial patch cables more,

Everyone Needs a Trainer… Get yours.
In the ever-evolving world of automation, particularly in biotech and pharma manufacturing, hands-on experience and continuous learning are invaluable. To

ISPE Vendor Night – Lucid Automation in San Francisco
Our engineering team had a great time at ISPE Vendor Night. Not only highlighting the latest technologies, but served as
Elevating Your HMI Designs
Elevating Your HMI Designs to Elite Status with my latest piece on Automation World . Discover the immediately actionable techniques we

ITOT Converge in Standard Server Rack
Our latest PLC install, smartly nestled in a 19″ server rack. The image speaks volumes about Lucid Automation’s commitment to

Leverage Custom Bioreactor Automation
Leverage the long equipment lead times with custom bioreactor automation to streamline your bioreactor processes. Regardless if you’re still in

Process Data Lacking?
Automation gains are often realized on the back end of a project. Having a strategy upfront increases the effectiveness, cost, and

New Equipment: Deciding on Custom vs. Default Automation
You’re specifying new bioreactor equipment. Time to consider automation options: Should you buy the off the shelf automation package or

chatGPT Generated PLC Code
Functional PLC Structured Text Generation Using chatGPT: Yes, it Works Below, I detail how, within a few hours of learning

Non-Obvious Gains Through Automation Strategy
Automation gains are often realized on the back end of a project. Having a strategy upfront increases the effectiveness, cost, and

Lucid Supports “Girls In STEM” at UC Davis
https://www.instagram.com/reel/CuADSo-Lu1A/?utm_source=ig_web_copy_link&igshid=MjAxZDBhZDhlNA== Big shout out to Marie and Haruka for participating in the STEM For Girls event at University of California,

Lucid Expands To Novato and Davis California
We’re proud to be expanding our GMP Life Science Automation operations in California. Our second office is up and running

Switching to Single-Use Purification
Switching to Single-Use Down Stream Purification, either Chromatography or Tangential Flow Filtration, is not as straight forward as the marketing

Single-Use Chromatography Data Analytic Upgrades
Life is too short to suffer bad automation. Upgrading off-the-shelf chromatography skids is a cost-effective method to increase productivity and close

Validating GMP AI Models Published on Automation World
Automation World published my article on validating control system ML models. It’s an exciting new area for life science automation,
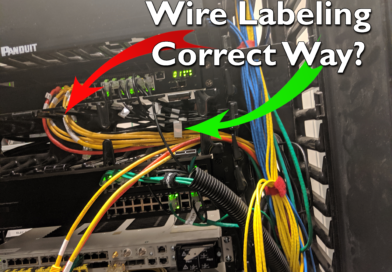
Wire Label
There is one optimal way to label a wire: length-wise, tight to insulation, and near point of termination. Yet in
Tuning VMs For Industrial Applications
Virtual machines are used at every level because of the value they provide in development, testing, backup, and recovery. But
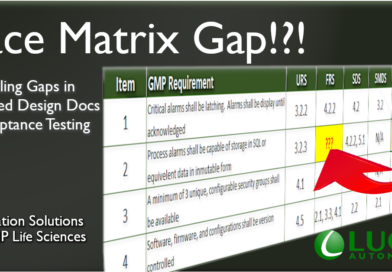
Trace Matrix Gap
Inevitably, we all run into a documentation gap. Restarting the workflows to initiate the change, approve, test, and review can

Alarm Management (ISA 18.2) – Automate This! Podcasts
How many nuisance alarms do you have active? Alarm management includes cheap alarms, ISA 18.2, code libraries, and release by

Retrofitting Stratix 5700 into Existing Biotech Skid
Retrofitting an OEM skid with a Stratix 5700. When buying new equipment be sure to specify, at a minimum, an


