Automation gains are often realized on the back end of a project. Having a strategy upfront increases the effectiveness, cost, and
Data Integrity & Analytics
Process Historian
Historical Data Collection, Analysis, Timely Reporting, and Audit records to stake holders is the core of regulated biopharma industry.
We have your Data Integrity solutions leveraging OSI Pi, FT Historian, InSQL, or customized solution to your use.
Scalable Database
Robust, secure, and scalable database architectures to address the wide application of data collection, analytics, and consolidation.
Extend the life and power of legacy systems with consolidating and optimizing of data retrieval by migrating to current platform. virtualization and thin clients.
Asset Management
Facility-wide and enterprise-wide views into asset performance, unplanned shutdowns, and optimization of maintenance resources can help increase production.
Provide data that informs and improves efficiency, reliability, and safety.
We understand critical data. Data that is worth just as much as the product you’re manufacturing. Whether storing it in OSI Pi, Rockwell FT Historian SE, Microsoft SQL, or some other database, we’ll be sure the integrity of your data is maintained from acquisition, to transfer, to at rest.
Once the data is safe, we’ll help you analyze and create actionable reports with SSRS, Rockwell VantagePoint, Dream Reports, Chrystal Reports, or Power BI.
Data Integrity Posts

Single-Use Chromatography Data Analytic Upgrades
Life is too short to suffer bad automation. Upgrading off-the-shelf chromatography skids is a cost-effective method to increase productivity and close
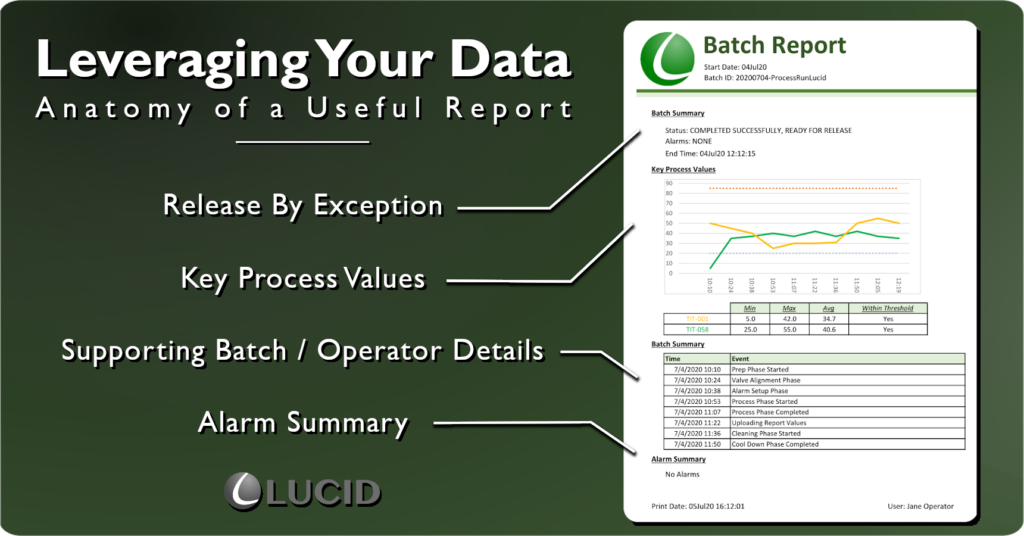
Release By Exception – Leverage Your Data
The most satisfying report I ever wrote had one, plain-text “COMPLETED SUCCESSFULLY, READY FOR RELEASE” line distilled from 5k+ batch
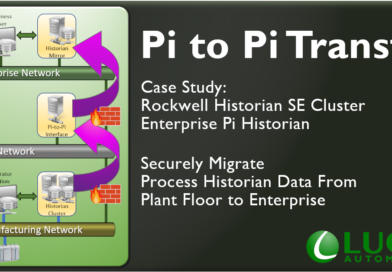
Pi to Pi Historian Transfer
Some tips and performance metrics for a project we just completed replicating a Historian Collective from plant floor securely up

PLC to SQL, Why Would I?
https://www.linkedin.com/pulse/plc-sql-why-would-i-bill-mueller/ There exists a gap between the process historians and ODBC-enabled batch/SCADA services to get PLC tag data into a
Prepared To Visualize Your Data – RtReports
RtReports are built to address the needs of Biotech and Pharmaceutical Processes. There are many data visualization solutions out there,

Release By Exception – Leverage Your Data
The most satisfying report I ever wrote had one, plain-text “COMPLETED SUCCESSFULLY, READY FOR RELEASE” line distilled from 5k+ batch

Pi to Pi Historian Transfer
Some tips and performance metrics for a project we just completed replicating a Historian Collective from plant floor securely up

PLC to SQL, Why Would I?
https://www.linkedin.com/pulse/plc-sql-why-would-i-bill-mueller/ There exists a gap between the process historians and ODBC-enabled batch/SCADA services to get PLC tag data into a
Prepared To Visualize Your Data – RtReports
RtReports are built to address the needs of Biotech and Pharmaceutical Processes. There are many data visualization solutions out there,
Latest News

Everyone Needs a Trainer… Get yours.
In the ever-evolving world of automation, particularly in biotech and pharma manufacturing, hands-on experience and continuous learning are invaluable. To





